Celltrion and Teva File Declaratory Judgment Action Against Thirty-Seven Rituxan®-related Patents
We previously reported that on January 11, 2018, Celltrion, Inc., Celltrion
Healthcare, Co. Ltd. (collectively “Celltrion”), Teva Pharmaceuticals
International GmbH, and Teva Pharmaceuticals USA (collectively “Teva”) filed
suit seeking declaratory judgment that thirty-eight patents relating to
Herceptin® (trastuzumab) are non-infringed, invalid, or unenforceable. That same
day, Celltrion and Teva also filed a suit seeking declaratory judgment that
thirty-seven patents relating to Rituxan® (rituximab) are non-infringed and
invalid. Many of the patents named in this suit overlap with those named in the
Herceptin®-related action.
Celltrion and Teva’s rituximab suit names
Genentech, Inc., Biogen Inc., Hoffmann-La Roche Inc., and City of Hope as
defendants. According to Celltrion and Teva, a substantial controversy of
“sufficient immediacy and reality to warrant the issuance of a declaratory
judgment” exists between the parties due to Celltrion’s filing of an application
under the BPCIA that seeks approval to market a rituximab biosimilar product.
Teva is Celltrion’s exclusive partner for the selling and distribution of the
rituximab biosimilar in the United States.
According to the complaint,
the FDA accepted Celltrion’s biosimilar application on June 27, 2017, and since
that time the parties have been engaged in the so-called “patent dance” portion
of the BPCIA. In particular, on July 17, 2017, Celltrion produced its biosimilar
application to Genentech as well as “other detailed information regarding the
manufacturing processes used to make [the rituximab biosimilar].” On September
14, 2017, Genentech then provided a list of forty patents that it believed could
be reasonably asserted against Celltrion’s proposed biosimilar. The parties
exchanged detailed statements asserting the factual and legal basis for their
respective positions on infringement and validity of the patents identified by
Genentech. On January 11, 2018, Celltrion notified Genentech that it wished to
litigate all of the patents Genentech identified on its list, and filed a
declaratory judgment action.
A summary chart identifying the
patents-in-suit, the patents that overlap with the previously reported
Herceptin®-related suit, and Celltrion’s requested relief for each patent is
provided below: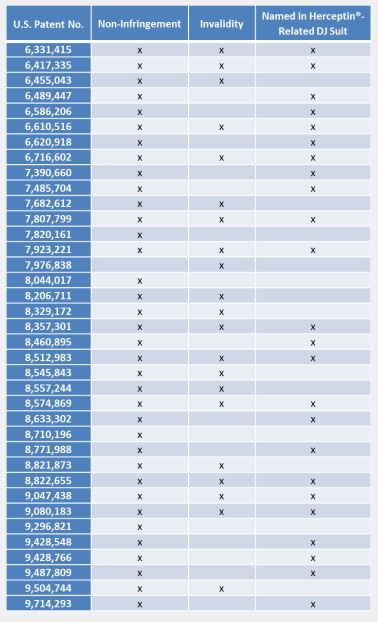
As we
previously reported, Celltrion filed a petition for inter partes review (“IPR”)
of the ʼ838 patent, but institution was denied. Similarly, institution of review
of the ʼ172 patent, the ʼ244 patent, the ʼ612 patent, and the ʼ711 patent was
also denied. However, review of the ʼ161 patent and the ʼ821 patent was
instituted by the PTAB.
Rituximab is an anti-CD20 chimeric murine/human
monoclonal antibody approved for the treatment of non-Hodgkin’s lymphoma,
chronic lyphocytic leukemia, rheumatoid arthritis, granulomatosis with
polyangitis, and microscopic polyangitis.
-
Previous:
-
Next:






